The Sinclair lab is driven by the belief that humanity can do better and that everyone has the right to the best medical care and maximum lifespan, no matter their gender, social status, or age. Work by our lab and others has shown that the pace of aging is not inexorable or predetermined, but rather can be slowed and even reversed by a variety of approaches. These include activating the body’s defenses against aging, deleting senescent cells, and reprogramming cells in vivo. In doing so, we can protect the body against and treat both rare and common diseases including mitochondrial diseases, type 2 diabetes, Alzheimer’s disease, cardiovascular disease, and cancer.
EXAMPLES OF PROJECTS IN THE LAB:
Is aging loss of digital or analog information? Is epigenetic noise the reason we age?
We have developed “The Relocalization of Chromatin Modifiers (RCM) Hypothesis,” which proposes that epigenetic changes due to relocalization of chromatin factors in response to DNA damage may be a chief cause of aging. This is true for yeast cells and it appears to be true for all eukaryotes. In response to a DNA break, proteins that regulate gene expression move to the break to help repair, resulting in gene expression changes. In young cells, this process is reset upon completion of DNA repair. However, not all proteins make it back to where they came from, which leads to gene expression changes and a loss of cell identity. We have developed the “ICE” mouse (for inducible changes in the epigenome), which allows us to induce DNA breaks and drive epigenetic changes that accelerate aging. Work is now centered on reversing this aging process.

Reprogramming cells to be young again
Our work has led us to the conclusion that the loss of epigenetic information is likely the root cause of aging. By analogy, if DNA is the digital information on a compact disc, then aging is due to scratches. We are searching for the polish. Our work has led us to identify reprogramming factors that we believe will enable us to reset a cell’s epigenetic status and reverse its age. We have developed human-compatible viral vectors to deliver the reprogramming genes to specific tissues or the entire body, thereby causing cells to act younger and wounds to heal faster. Our current focus is on nerve regeneration and the reversal of other symptoms of aging. We see treatments being possible for companion animals and humans to dramatically improve their health and lifespan.
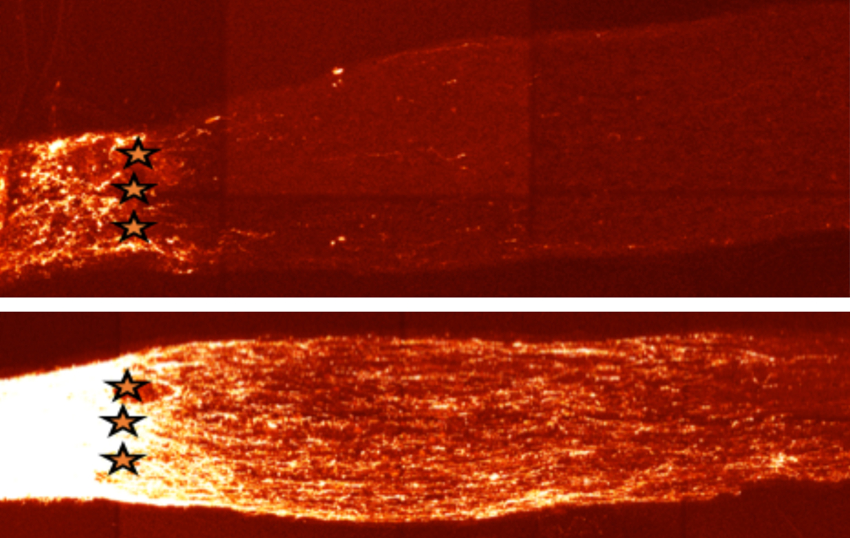
Can we develop drugs that slow aging?
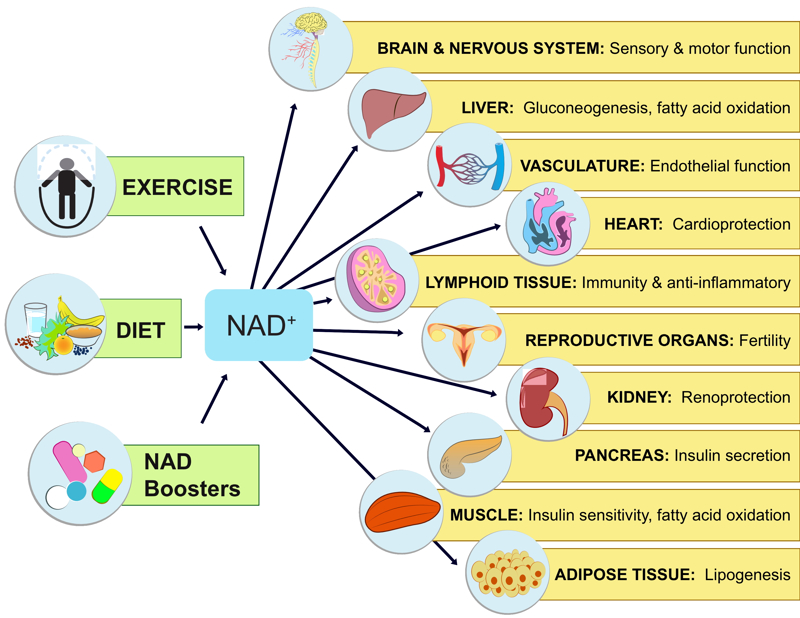
Our work on SIRT1 led us to an exciting finding that the level of nicotinamide adenine dinucleotide (NAD+), cofactor of SIRT1, declines with age. We study the mechanisms by which the NAD+ level affects DNA repair and look for therapeutic targets to improve this process. In particular, we focus on delineating the biology of NAD+-depleting and producing enzymes as direct tools to control the NAD+ level in the cells toward increased health-span and improved physiological resilience.
The discovery of longevity genes showed that it is possible to greatly slow the pace of aging and disease by manipulating just one central pathway. This raises the possibility that we can find small molecules that can treat multiple, seemingly unrelated diseases, with a single medicine. Our lab has been highly active in this area, starting with the discovery of sirtuin activating compounds (STACs) in 2003. Since then, potent activators have been discovered and some of these are now in clinical trials, producing positive results. We have active studies to understand how STACs work at the molecular and the physiological levels using cutting-edge enzymological and structural methodologies and mouse genetic models in which we can delete genes at any time throughout the lifespan of the animal, and in specific organs. We published, for example, that the ability of resveratrol and a STAC called SRT1720 to increase mitochondrial function, require the SIRT1 gene in vivo. We have an active program to develop novel molecules that raise NAD levels. We are testing them for their effects on aging and age-related diseases. Human clinical trials with NAD-boosting molecules are ongoing.
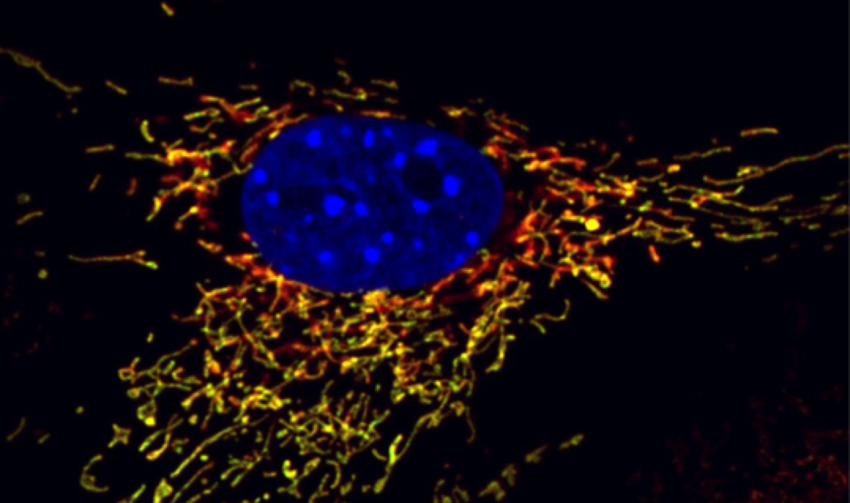
Understanding the role of mitochondria in aging and disease
The study of mitochondria has experienced a renaissance in recent years. A large body of evidence indicates that common aging-related diseases have a mitochondrial component. Yet, surprisingly little is known about what leads to the progressive loss of mitochondrial fitness during aging. We investigate the cellular mechanisms that could be employed to maintain the mitochondrial homeostasis and ultimately prolong health-span.
One of our research avenues led to the discovery of ongoing asynchrony between the nuclear and mitochondrial genomes during aging. Utilizing novel genetic and pharmacological approaches, we are using this knowledge to restore metabolic function in aged mice back to youthful levels. We established that mitochondrial NAD+ levels dictate cell survival, which we refer to as the “Mitochondrial Oasis Hypothesis.” Following up on this work, we have formulated an exciting hypothesis that leakage of NAD+ from mitochondria is a cause of aging and memory loss.
We are also interested in identifying new genes and signaling cascades in the human genome that control mitochondrial function. We are developing novel genome mining algorithms, using advanced sequencing and proteomics tools, and high-throughput screening methods to map the most complete network of mitochondrial regulators. This work will provide new insights into fundamental aspects of mitochondrial biology and how mitochondrial defects may be prevented or corrected. We are also interested in finding novel secreted factors that increase mitochondrial function and are candidates for signaling factors that have recently been implicated in the systemic control of aging in simple organisms.
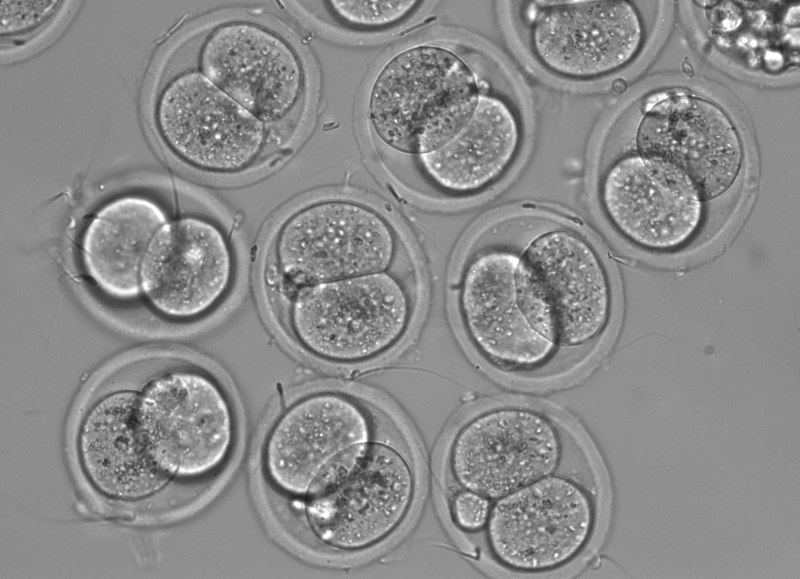
Delaying menopause and reversing female infertility
Ovarian stem cells are a recently discovered type of cell than can give rise to oocytes in culture and produce healthy oocytes in vivo. This work may overturn the dogma that a female is born with a set number of eggs that are simply lost over time due to damage and genomic instability. We are using our knowledge gained from studying aging and metabolism to understand how female infertility may be delayed or reversed. Our goals are to identify genes and small molecules that can reactivate ovarian stem cells in vivo to treat premature ovarian failure, chemotherapeutic ovarian failure (in cancer patients) and extending the healthy and fertile period for women.
Can we slow down or even reverse neurodegenerative diseases?
Neurodegenerative diseases strike primarily in mid to late life, and thus their incidence rises in aging populations. We are actively working on identifying the molecular drivers of neuronal degeneration such as novel genes, epigenetic changes and metabolic imbalance by applying diverse experimental approaches including classical studies of genes and gene function, advanced omics and novel transgenic mouse models, including the NICE mouse (for neuronal inducible changes in the epigenome) which allows us to study the effects of epigenetic changes in the aging brain. Through our studies we aim to develop therapeutic interventions that can prevent the onset or slow the progression of disease, and possibly even reverse it by regenerating the damaged tissues.
Uncovering the human secretome
Peptide hormones regulate embryonic development and most physiological processes by acting as endocrine or paracrine signals. They also hold great therapeutic potential either as medicines or targets for treating both common and rare diseases. Yet identifying peptide-coding genes below ~300 base pairs is inherently difficult because they exist within the “genomic noise”. Our goal is to uncover the human secretome and use newly discovered hormones to improve the human condition. Over the past few years, we have developed a unique pipeline of technologies that combines breakthroughs in math, computer hardware and software, proteomics, mass spectrometry, and high-throughput screening, each of which has been optimized and integrated. Using this platform, for which we have been awarded an NIH Director’s Pioneer Award, we have discovered thousands of putative peptide-coding genes. Our aim is to screen these peptides for activities to determine their biological roles and potential therapeutic application in biology and disease settings.